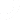The burgeoning vape industry is about to transform side by side with its exponential rise. As with any new emerging industry, there are always regulations that need to be updated or created in order to better serve its community and the public. Bill S-5 is aimed at revising the Tobacco Act to include provisions on the sale and production of vaping products in Canada. It will also make changes to the Non-smokers’ Health Act and consequential amendments to other Acts. These adjustments include new governance over the manufacture, sale, labelling and promotion of vaping products. In order to combat these new regulations a significant number of changes will need to occur in the way manufacturers operate and manage their quality standards. So, what are these changes and how can you be ready for them?
4 Key Changes Bill S-5 Will Make In Your Quality Management:
Labelling:
- Requirement that vape products or product packages clearly show consumer awareness information as health warnings, nicotine concentration, and a list of ingredients.
Reporting & Public Disclosure:
- Vape product manufacturers must regularly report ingredients, sales, promotion and research activities information to the Minister of Health.
- Both the manufacturer and ministry are responsible for making public certain information on vape and tobacco products.
Health & Safety:
- Amendments to the Canada Consumer Product Safety Act for its requirements to address the hazards, such as electrical, mechanical, and toxicological hazards, posed by those vaping products not marketed for a therapeutic purpose.
Strengthened Compliance & Enforcement Authorities: Compliance and enforcement authorities in the Tobacco and Vaping Products Act will combine with those found in other modern statutes. This will includes the Canada Consumer Product Safety Act. Mandates for regulatory processes such as GMP are likely to follow and measures to inspect and ensure their adoption will be employed. Some authorities include:
- Inspectors using tele warrants in carrying out an inspection.
- Allowing inspectors to enter or pass through private property (other than a dwelling or house) to carry out an inspection.
- The accompaniment of any person necessary to conduct the inspection (e.g. a scientific expert) with the inspector.
- Requiring manufacturers to keep records.
- Inspection by stopping or moving a means of transportation.
- Recovering costs for the storage or destruction of seized goods.
Using Isolocity To Your Advantage
Isolocity’s quality management system anticipates changes in legislation, just like these! Reporting and compliance is all managed easily within our “Inventory Management” module. You can oversee inventory changes right from your dashboard or use the inventory toolbar from the module to view graphical breakdowns of your inventory/production levels. Adding or subtracting parts, shipments, or ingredients are a cinch since Isolocity will automatically update levels in the system.
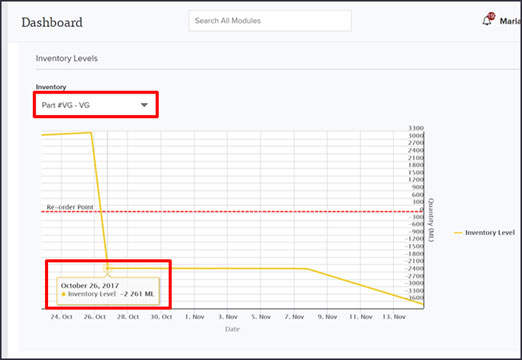
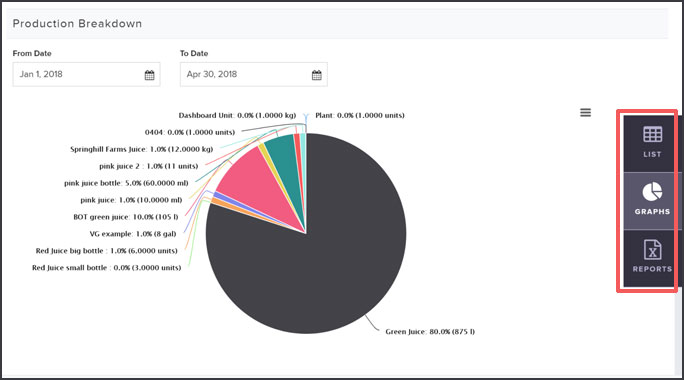
Create reports by simply clicking on the “Reports” view in the toolbar. Here you can download the full inventory and production reports required by the Minister of Health in Bill S-5.
Rate us on G2.