Introduction
When we think of a 21 CFR 820 Nonconforming Product, the first thing that comes to mind is a consumer who has been injured by a defective product. But did you know that many nonconforming products are not defective? The term “nonconformance” refers to any deviation from the requirements of 21 CFR 820.
This means that with every shipment that arrives at your warehouse, there’s a chance it will be rejected because it doesn’t meet your standards or specifications. When this happens, how should you handle these items? How can you ensure they don’t make their way into the marketplace?
What is a 21 CFR 820 Nonconforming Product?
- Nonconforming Product: A nonconforming product is any product that is not in compliance with the requirements of the product specification.
- Defective Product: A defective product is one that was manufactured in a way that violates safety standards or fails to perform its intended purpose.
- Substandard Product: A substandard product is something that doesn’t meet standard quality levels for its intended use.
Nonconformity can occur during all stages of production: design process; pre-production inspection; production process; post-production inspection; packaging/storage; shipping/delivery; installation
Process flow for 21 CFR 820 Nonconforming Product
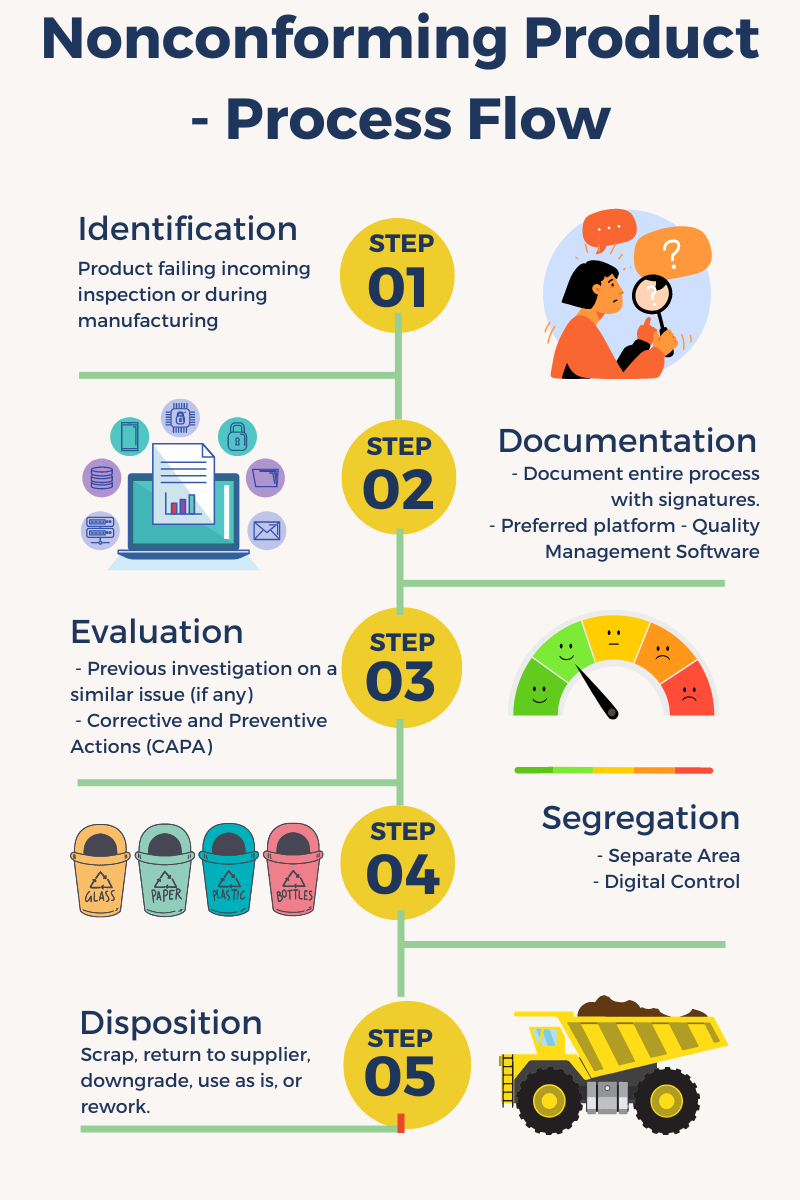
21 CFR 820 Nonconforming Product
What is the classification of a 21 CFR 820 Nonconforming Product?
As with any regulatory term, there are a lot of ways to define a nonconforming product. But the most common one is a categorization based on risk level and how much testing needs to be done before the product can be sold. This classification system is called 21 CFR 820:
-
Class I (lowest risk) – No testing is required
-
Class II – Acceptable reportable products that require no more than visual examination only (e.g., toys)
-
Class III (medium risk) – Acceptable reportable products that require some additional testing or evaluation prior to sale (e.g., detergents)
-
Class IV through X (highest risk) – Highly toxic and carcinogenic substances such as radioactive materials used in medicine or research labs
Who is responsible for disposing and handling nonconforming products?
When it comes to disposing and handling nonconforming products, the manufacturer of the product is responsible for ensuring that it does not cause harm to humans or the environment. The distributor and importer of said product are also responsible for ensuring that no damage occurs.
If you are a manufacturer, distributor, or importer and have received a recall notice for violating 21 CFR 820, it is crucial to ensure the return of all products under your control to your company for appropriate disposal.
If immediate action is not feasible due to insufficient time before the FDA deadline, you have the option to request an extension from federal regulators. This is to avoid unnecessarily jeopardizing consumer safety.
How should you segregate the nonconforming product?
You should keep the nonconforming product separate from the rest of your products. Ensure compliance by keeping the nonconforming products in a specifically designated area or storing them in a designated container solely assigned to them. Avoid using this area or container for other products to prevent any potential mix-up or confusion.
When should you reject a nonconforming product?
As a manufacturer, you must reject a nonconforming product if:
- It presents a health or safety hazard.
- It does not meet legal requirements.
- It violates regulations.
- It is in violation of contract specifications.
How to handle rejected products to prevent their entry into the market
- Destroy rejected products.
- Dispose of rejected products appropriately.
- Document the disposition of rejected products according to applicable requirements (e.g., Federal Food, Drug, and Cosmetic Act).
- Segregate rejected products from the nonrejected products until disposal is complete or further processing is required by law or regulation.
- Track all actions taken with respect to the disposition of a rejected lot as appropriate for your facility (e.g., print labels; place them into shipping containers). Test all remaining inventory against specifications at least annually (or more often if necessary) to ensure that no other subpar lots exist within your inventory system and are not entering into commerce.*
What proactive actions can companies take to address nonconformance?
Let’s look at some examples of what companies can do to address nonconformance.
- Talk with your customers and ask them what they think your company could be doing better right now, or where they think the biggest problems lie in their experience with your products or services. Use surveys, focus groups, interviews, etc., depending on how large or small of a number of people you want to talk to
- Look at data from past products/projects and identify trends that suggest there may be issues with quality (e.g., if every product that’s been shipped has been returned due to defects).
3 regulations related to 21 CFR 820.90
- 21 CFR 820.1 encompasses the FDA’s overall regulations for drugs and biological products. It includes details about the specific requirements applicable to each type of product, the conditions for their distribution in interstate commerce, and the labeling requirements.
- 21 CFR 820.20 outlines general quality requirements for drug production. These requirements encompass measures to prevent the contamination of starting materials by microorganisms, including viruses. The regulation also covers aspects such as environmental monitoring, the establishment of a quality control unit, the implementation of written standard operating procedures, reporting procedures (including deviation reports), development of corrective action plans, establishment of complaint handling systems, and maintenance of training records for personnel involved in manufacturing operations.
- Regarding 21 CFR 820.90, it pertains to medical devices that have not undergone FDA testing or clearance. The regulation mandates that such devices must be labeled with all essential information regarding their performance characteristics or limitations. This includes any restrictions on use or warnings related to potential adverse events.
Keep in mind that when it comes to 21 CFR 820, your action regarding nonconforming products can impact your company.
It is crucial to note that your actions concerning nonconforming products under 21 CFR 820 can have significant consequences for your company. Failing to comply with the requirements of 21 CFR 820.100 and subsequently experiencing a product recall following an FDA or regulatory agency inspection can lead to potential civil or criminal penalties.
To mitigate such risks, it is essential to comprehend the available options for nonconforming product reporting and take appropriate measures accordingly.
We have also come across this PDF from the FDA website, check this out, it is very helpful.
Conclusion
The bottom line is that when it comes to 21 CFR 820, your actions play an important role in determining the future of your company. If you need help with Nonconforming products, Isolocity can definitely help. Get in touch with us and we will give you a live demo of how our software can help.