The Quality
Management Blog
Latest posts
Filter
EventsResources
3 reasons to attend the Quality show south by Quality Magazine
As a brand-new event, the Quality Show South might raise a few eyebrows, especially considering…
April 17, 2024Food Safety
FDA’s latest update on Food Safety Modernization Act Section 204 (FSMA 204)
In our latest blog, where we discussed our experience and thoughts on SQF Unites, we…
April 5, 2024EventsFood SafetySQF
SQF Unites: Is it worth the time?
SQF Unites 2024 was a great success for Isolocity. Attending this conference was vital for…
March 28, 2024Food SafetyHACCPSQF
What are the differences between HACCP and SQF?
If you're passionate about food safety, chances are you're familiar with the HACCP and SQF…
March 7, 2024Food SafetyHACCP
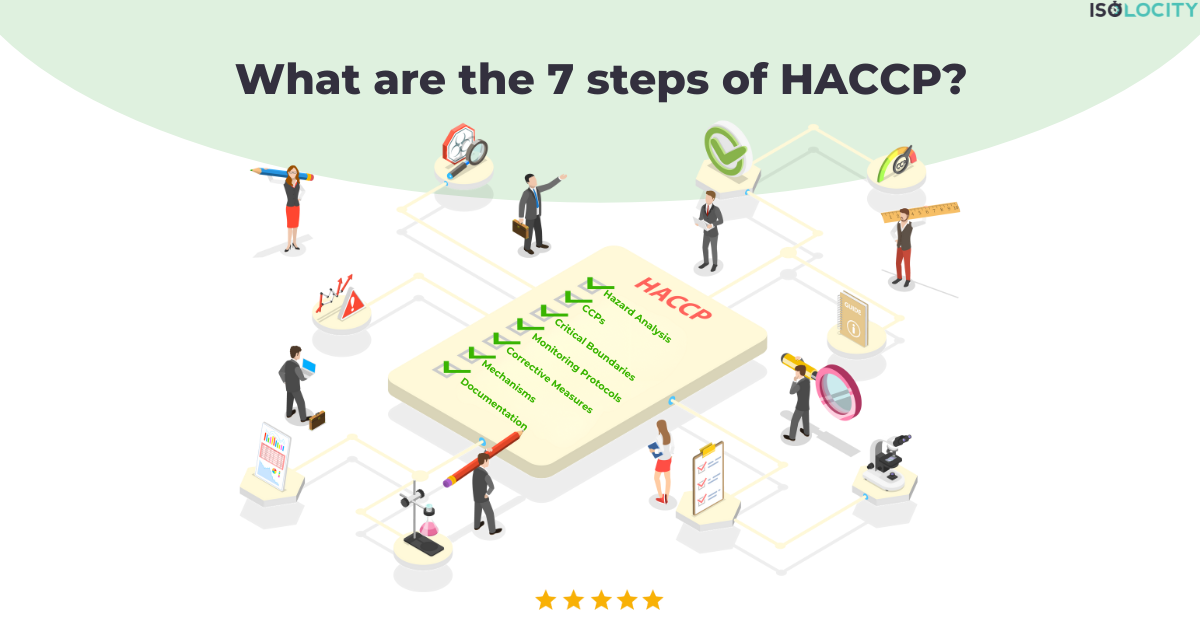
What are the 7 steps of HACCP?
The Hazard Analysis and Critical Control Points (HACCP) system is a leading example of systematic…
March 5, 2024Food SafetySQF
What are the 3 Principles of the SQF Certification?
Ensuring quality is crucial in all aspects of life. Whether it's purchasing furniture or booking…
February 29, 2024-

April 17, 2024Events, Resources
3 reasons to attend the Quality show south by Quality Magazine -

April 5, 2024Food Safety
FDA’s latest update on Food Safety Modernization Act Section 204 (FSMA 204) -

March 28, 2024Events, Food Safety, SQF
SQF Unites: Is it worth the time? -

March 7, 2024Food Safety, HACCP, SQF
What are the differences between HACCP and SQF?