What is a Software as Medical Device (SaMD)?
According to 21 CFR 862 or 866, a SaMD is software that has received FDA approval as a medical device or as a CLIA-waived device. A SaMD might contain digital tools like electronic health record systems that assist AME workflow procedures like reading patient reports or gaining access to electronic medical records, or mobile applications that allow data sharing.
Latest News:
- Marzio Ghezzi, CEO of Mia-Care, highlights the growing trend towards the integration of data-driven and patient-centered care. Read more at med-technews.
- Digital-first Software as a Medical Device (SaMD) is revolutionizing healthcare. Check out the article written by Med Tech Intelligence.
- The market for software as a medical device (SaMD) is expected to expand at a rapid rate during the period 2023-2028 as per the report shared by 360 Research Reports.
Not fond of reading? Watch this video.
SaMD – eligibility for software
SaMD includes software that satisfies all three requirements;
- It includes particular elements called for by regulatory bodies for safety and efficiency.
- In accordance with the necessary rules and regulations, it must undergo testing and certification by an approved agency.
- The producer must offer proof that they adhere to these rules.
Classifications of SaMD
The International Medical Device Regulators Forum (IMDRF) has provided a framework for classifying SaMD. It is based on their risk level, intended use, and potential impact on patient health. The five categories of SaMD are as follows:
- Class I: SaMD with a low risk to the patient and/or user. It is typically employed for promoting or maintaining general health and wellness, and not for diagnosing or treating medical conditions.
- Class IIa: SaMD with a moderate risk to the patient, used to monitor and diagnose medical conditions or to support medical decisions.
- Class IIb: SaMD with a higher risk compared to Class IIa, used for monitoring and diagnosing severe medical conditions or supporting high-impact decisions.
- Class III: SaMD with the highest risk to the patient, used for diagnosing, monitoring, or treating critical or life-threatening medical conditions in which an incorrect result could result in severe health consequences or mortality.
The origin of SaMD
HIMSS (Healthcare Information and Management Systems Society) created the term SaMD in 2009. It designates software that is a component of a medical device or system but does not fit the criteria of an electronic health record (EHR). Because it’s not an EHR and doesn’t gather patient data or provide users access to their own medical information through the app itself. A SaMD would be, for instance, an app that lets users track their blood pressure over time.
Software as a medical device (SaMD) is software that replaces or supports the functions of a medical device
Even though you don’t need to physically connect the software to the patient, the SaMD can perform the same tasks as its hardware equivalent. This implies that you may use it on a variety of devices, such as tablets or smartphones.
The key benefit of adopting this kind of program is that it enables real-time health monitoring from any location with an internet connection.
Expected growth of Software as a medical device (SaMD)
In the upcoming years, the rise of SaMD would continue to pick up speed. From 2021 to 2028, it may expand at a compound annual growth rate (CAGR) of 25.6%. The expanding acceptance of mobile health (mHealth) solutions, the prevalence of chronic illnesses, and the demand for affordable healthcare solutions are some of the drivers that are fueling this rise.
The COVID-19 pandemic contributed to the rise of SaMD. The healthcare sector turned to telemedicine and distant care to lower the risk of infection transmission. The ability to remotely monitor patient health and deliver prompt treatments makes SaMD technologies well-suited for remote care.
Technological improvements, rising patient demand for individualized, patient-centered care, and the need for affordable, effective healthcare solutions will all contribute to the expansion of SaMD. Regulatory difficulties and worries over data privacy are two obstacles, though.
How can I get a software license from the US Food and Drug Administration (FDA)?
The good news for US-based developers is that obtaining a software license from the FDA is not too difficult. What you must do is as follows:
- Become a maker of electronic medical devices (EMDM). You can do this by submitting a registration form online or by submitting Form FDA 2532a via the Medical Device Reports System of your state (MDR). You should register as soon as possible if your product is already on the market. Patients or healthcare professionals should not use it without the FDA’s official permission.
- If your product does not already have an ID number assigned by the FDA, request one.
What documents do I need to apply for a software license from the FDA?
Depending on the kind of medical device you will use with your software, you will require different documentation to apply for a software license with the FDA. For instance, if it’s an implanted device, you’ll need to include details on its design, operation, and safety and efficacy information. If it’s not an implanted device and doesn’t need pre-market clearance or notice, you simply need to submit information on the device’s design and function.
Who can apply for a software license from the FDA?
Yes, however, it depends on your circumstances. Small businesses without venture money or other support will very certainly have to submit their licensing applications. If this is not the case and you have access to grants or investments, some businesses can speed up the procedure by doing all of the paperwork and research for you.
Be sure the agency is reliable if you decide to use them rather than doing it yourself. Several frauds out there take advantage of folks who lack knowledge or free time (like me).
How long does it take to get a medical device license?
The FDA issues medical device licenses, which are necessary to commercialize your product in the US. The following factors affect how long it takes to obtain a medical device license:
- The kind of device you’re building. For e.g., a Class II medical device is easier to acquire an IDE than a Class III
- The advanced clinical study design suggested
- Regardless of whether you have previous expertise managing clinical studies utilizing comparable devices or technology
What if my software is considered as SaMD but doesn’t have a software license from the FDA?
If your software is classified as SaMD, but you don’t have a software license from the FDA, you must stop selling it right away to avoid legal repercussions. Before enforcement begins, there could be a grace period. However, you can only ascertain this by communicating directly with the FDA.
SaMDs are regulated as medical devices
As SaMDs are considered medical devices, the FDA must authorize their use in customary clinical procedures. You must submit a premarket approval (PMA) application to obtain permission for your SaMD. Your application must contain details about how you created the device and why it is secure and efficient. The FDA will examine this data before making a decision about the approval of your product.
Frequently Asked Questions about SaMD
Q1: Do patients use SaMD at home or in hospitals and clinics?
A: Patients can use SaMD at home or in clinics, depending on its intended use and regulatory classification.
Q2: Does SaMD always need an internet connection?
A: No, not every time. SaMD can work without an internet connection, but some may benefit from it for data sharing and updates.
Q3: What data security steps should SaMD developers take to protect patient information?
A: To keep patient data safe, SaMD developers should use strong encryption, set limits on who can view the data, and do regular security checks.
Q4: Can SaMD work with other medical tools or EHRs (electronic health records)?
A: SaMD can be linked to EHRs and other healthcare systems. That makes it easier to share information and improve patient care.
Q5: Do standards for post-market tracking apply to SaMD products?
A: Yes, SaMD makers often need to keep an eye on how their products work and let regulatory bodies know about any problems.
Q6: Do any global rules and laws for the development of SaMD exist?
A: Yes, organizations like the IMDRF and ISO have set worldwide standards for the development and regulation of SaMD.
Q7: What would happen if SaMD was cleared but later found to have safety issues?
A: If safety problems are found with SaMD products that have been approved, regulatory bodies may issue recalls or ask for fixes.
Q8: Can SaMD be bought overseas, or does it have to be approved in every country?
A: Most of the time, developers of SaMD have to get permission or follow the law in every country where they want to sell their products.
Q9: Can SaMD give people their own treatment plans and medical care?
A: Yes, SaMD’s study of patient data to customize treatment plans and treatments can help with personalized medicine.
Q10: Are tax breaks or funds available for SaMD developers?
A: Some places offer tax breaks or subsidies to support innovation in the healthcare technology field, especially in the area of SaMD development.
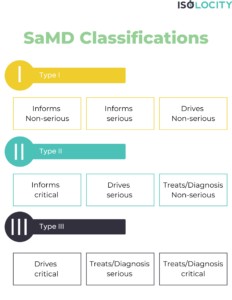
Q11: What are the examples of SaMD?
A:
- Type 1 (Low Risk): Weight management mobile app that provides general advice on diet and exercise without making critical health decisions.
- Type 2 (Moderate Risk): Software analyzing medical images for detecting common conditions, such as bone fractures, with guidance for healthcare professionals on interpretation.
- Type 3 (High Risk): SaMD designed for the diagnosis of life-threatening conditions, like a software application that analyzes cardiac images to identify potential heart abnormalities, guiding critical medical decisions.
Q12: What is the difference between MDDS and SaMD?
A: Both Medical Device Data Systems (MDDS) and Software as a Medical Device (SaMD) play crucial roles in healthcare technology. However, they differ in their scope and functionalities. MDDS primarily focuses on the electronic transfer, storage, and display of medical device data, serving as intermediaries. On the other hand, SaMD goes beyond, actively collecting, and analyzing individual patient data to offer personalized insights and optimize healthcare outcomes. Transitioning from MDDS to SaMD represents a shift towards more sophisticated, patient-centric digital solutions in the evolving landscape of medical technology.
Conclusion
We hope you found this article helpful and informative. Keep an eye on this blog post as we will keep adding more information related to SaMD. For any inquiries, feel free to contact us.