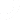In our latest blog, where we discussed our experience and thoughts on SQF Unites, we gave a small hint about the upcoming changes on Food Safety Modernization Act Section 204 (FSMA 204). It was a hot topic at SQF Unites, and in this blog, we are going to dig deeper into this topic.
We will try to keep this article as recent as possible. So we will keep updating all the latest information as we get access to them and will keep adding them here. So if FSMA 204 interests you, we suggest you bookmark this article, as it will keep getting new information.
A few days ago, the FSMA 204 page was updated on the FDA’s website. So this article is based on those updates. The FDA released the final rule of FSMA, Mandating Additional Traceability Records for Specific Food Items.
In a bid to fortify food safety measures, the FDA has introduced the Food Traceability Final Rule. Let’s break down this significant regulation in a simplified manner to understand its key components and implications.
Prefer AV over text? Check this video out!
1. Understanding FSMA 204
The FDA’s FSMA 204 Final Rule necessitates additional record-keeping for certain foods, going beyond existing regulations. This applies to individuals involved in manufacturing, processing, packing, or holding foods listed on the Food Traceability List (FTL).
View this post on Instagram
Food Traceability List
The FTL has mainly these items; for more details, check the FTL page of FDA.
- Cheeses, other than hard cheeses
- Shell eggs
- Nut butters
- Fresh items
- Cucumbers
- Herbs
- Leafy greens (regular and cut)
- Melons
- Peppers
- Sprouts
- Tomatoes
- Regular fruits and Tropical tree fruits
- Vegetables other than leafy greens
- Protein
- Finfish (Fresh, frozen, smoked)
- Crustaceans (Fresh, frozen)
- Molluscan shellfish, bivalves (fresh, frozen)
- Ready-to-eat deli salads
The rule stems from Section 204(d) of the FDA Food Safety Modernization Act (FSMA 204) and is a crucial part of the FDA’s New Era of Smarter Food Safety Blueprint.
2. Key Requirements for FSMA 204
Record-keeping:
Entities must maintain records containing Key Data Elements (KDEs) associated with specific Critical Tracking Events (CTEs) related to the listed foods.
Related webinar : Setting the Standard for Quality Record-keeping with Lean Principles
Timely Reporting:
Information pertaining to these events must be provided to the FDA within 24 hours or within an agreed-upon reasonable timeframe.
3. Implementation and Compliance Date
The compliance date for adhering to these record-keeping requirements is set for January 20, 2026. This uniform compliance date aims to ensure effective implementation across the supply chain.
4. Scope and Coverage
The final rule applies to both domestic and foreign firms involved in the U.S. food supply chain. It encompasses various stages, from manufacturing to consumption, ensuring comprehensive coverage.
5. Critical Tracking Events (CTEs) and Key Data Elements (KDEs)
CTEs and KDEs are the most important aspects of FSMA 204. The rule identifies specific CTEs, including harvesting, cooling, initial packing, shipping, receiving, and transformation. For each CTE, corresponding KDEs must be maintained to facilitate effective traceability.
Let’s give an overview and mapping of KDEs for each CTE.
FSMA 204 CTE and KDE mapping
CTEs |
KDEs |
Harvesting |
|
Cooling (before initial packaging) |
|
Initial packaging (RAC) |
|
First land-based receiver |
|
Shipping |
|
Receiving |
|
Transformation |
|
Traceability plan |
Notes: should be able to update the traceability plan as needed; need to keep the record of the old traceability plan for at least 2 years. |
Although it may appear extensive, upon closer inspection, only a select few Key Data Elements (KDEs) are reiterated throughout the entire process. The FDA emphasizes the importance of maintaining accurate records of these KDEs. Notably, details such as date, location, quantity and unit, along with descriptions of processes and products, hold paramount significance. In the event of complications, having an efficient system to promptly retrieve this information is essential for pinpointing the root cause of any issues.
6. Traceability Lot Code (TLC)
A Traceability Lot Code (TLC) serves as a unique identifier for traceability lots within the records of the firm. It must be assigned to relevant foods and linked to associated KDEs.
This code is important for FSMA 204 because it will be the main source of all information and traceability. Even though the FDA accepts both paper-based and electronic systems, just think of the trouble of tracking a code through a paper-based system.
With Isolocity you can have a unique identifier for both your products and your batch. Which means you can track each product, track the batch of that product and find the root cause when needed.
7. Traceability Plan
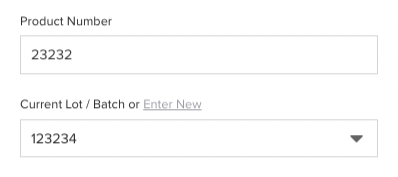
Entities subject to the rule must establish and maintain a traceability plan outlining procedures for record maintenance, identification of listed foods, TLC assignment, and designated points of contact.
Having an electronic QMS streamlines this process since the entire system is designed with compliance and traceability in mind. For instance, with Isolocity QMS, you benefit from a comprehensive audit trail. Therefore, if any issues arise, you can easily trace back to the source.
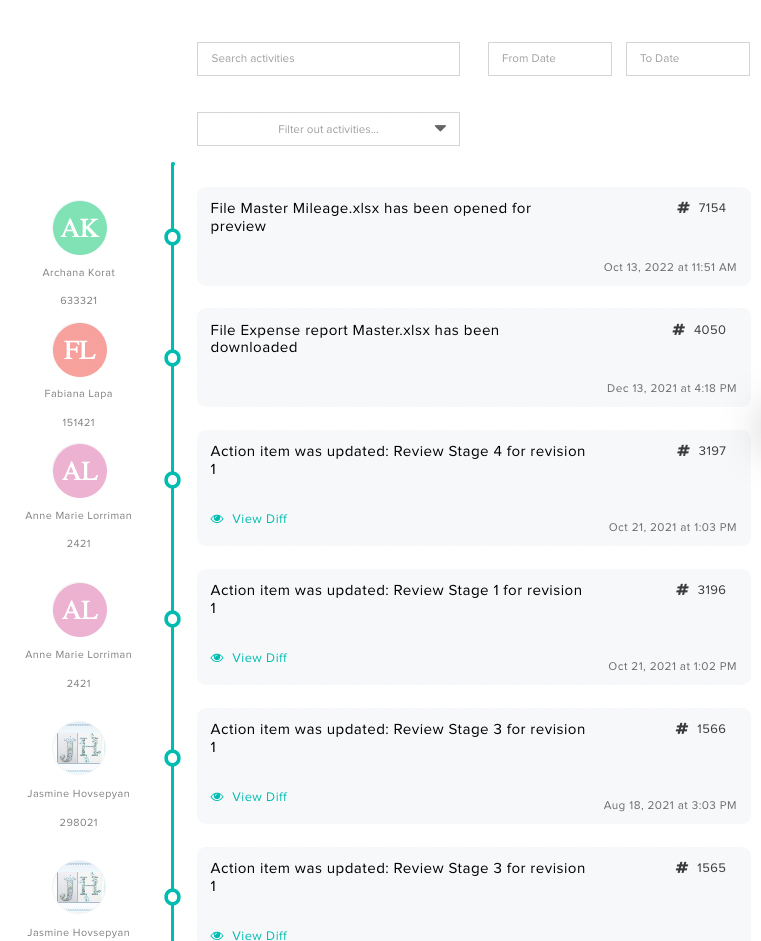
You can also track who signed or approved anything that has been processed.
These are merely two illustrations; the potential is limitless with an effective QMS such as Isolocity.
8. Record-keeping and Reporting
Records related to FSMA 204 must be maintained in either paper or electronic format, ensuring legibility and preventing deterioration. Compliance necessitates providing requested information to the FDA within specified timeframes.
We strongly suggest that you maintain an electronic format for the below reasons.
- With these new changes, audit trails play an important role, with a paper-based system that gets tricky.
- If anything goes wrong and the FDA asks you to submit some details, you are expected to submit them within 24 hours. Unless you are a small local player with one site, this is almost impossible with a paper-based system.
- All the different KDEs are interlinked and you would want a system that can automatically link these KDEs and let them travel throughout the system. A manual process with a paper-based system is extremely inefficient for this.
- The FDA requires you to retain the previous traceability plan for a period of two years. With a paper-based system, managing this task can quickly become overwhelming, involving the storage of thousands of documents for an extended period of time. It might even necessitate hiring a team of security guards to ensure their safety. Alternatively, opting for an electronic quality management system can offer a more efficient solution.
9. Exemptions, Modifications, and Waivers
Certain exemptions and partial exemptions exist, and entities may petition for modifications or waivers based on unique circumstances or economic hardship.
10. Simplifying Compliance
A simplified compliance process involves assessing applicability, understanding CTEs and KDEs, developing a traceability plan, and collaborating with supply chain partners.
Conclusion
The FDA’s Food Traceability Final Rule marks a significant stride in enhancing food safety measures. By enforcing robust record-keeping and traceability practices, it aims to expedite the identification and removal of potentially contaminated foods from the market, thereby reducing food-borne illnesses and fatalities.
If you believe there’s ample time for FSMA 204 compliance until the deadline of January 20, 2026, think again. It’s not solely about drafting documents; it’s about establishing a comprehensive process, which requires significant time investment. In reality, the timeline should be considered as yesterday, meaning there’s already been a day’s delay. Based on our experience, while product adoption may occur swiftly, training the entire organization and implementing an efficient process is a time-consuming endeavour.