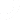The Medical Device Single Audit Program (MDSAP), enables auditing organizations recognized by the MDSAP to conduct one regulatory audit or medical device manufacturers that satisfies the requirements associated with the participating authorities of the program.
Who Are the International Partners That Participate in the MDSAP?
There are:
- Members
- Official observers
- Affiliate members
MDSAP members include the U.S. Food and Drug Administration (FDA), Health Canada, Therapeutic Goods Administration of Australia, and other organizations.
Official observers of the MDSAP include the European Union (EU), and the World Health Organization (WHO) Prequalification of In Vitro Diagnostics (IVDs) Programme.
There are also MDSAP affiliate members that include the Republic of Korea’s Ministry of Food and Drug Safety and Argentina’s National Administration of Drugs, Foods, and Medical Devices (ANMAT).
Why Is MDSAP Important?
MDSAP is important because it addresses the rapidly expanding healthcare technology innovations and global trade in the medical device sector. Today, medical manufacturers face many new challenges regarding medical device manufacturing. Thanks to the MDSAP, the audit report suffices as a form of routine agency inspection, which means multiple inspections are not required.
What Are the MDSAP Audit & Certification Requirements
The Starting Point
To become MDSA certified, it’s in your best interest to review the MDSAP FAQ. The FAQ is your starting point and it provides answers to many general questions you may have regarding the MDSAP, assessments, and audits, and heath Canada medical device regulations.
Implementation
The implementation process is when you discuss timeframes and costs with certified auditing trainers. You can decide to perform an optional pre-assessment, then start the gap analysis.
Certification
A stage one audit occurs during the certification process. When the stage one audit is complete, a detailed second stage audit must be performed. Once both are successful, the company receives certification. Once certification is complete, regulatory notification of successful completion of the audits and certification must be reported.
Maintaining Certification
Once certification is awarded, you need to maintain your certification. To properly maintain MDSA certification, annual surveillance audits need to be performed. You will need to recertify to maintain MDSAP status every three years. Another part of maintaining your MDSA certification is establishing a continuous improvement culture.
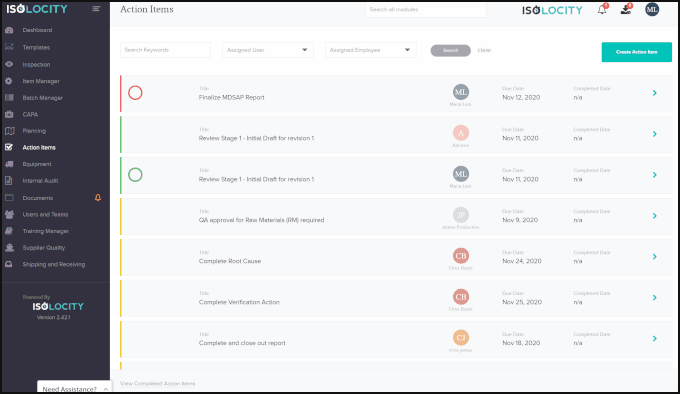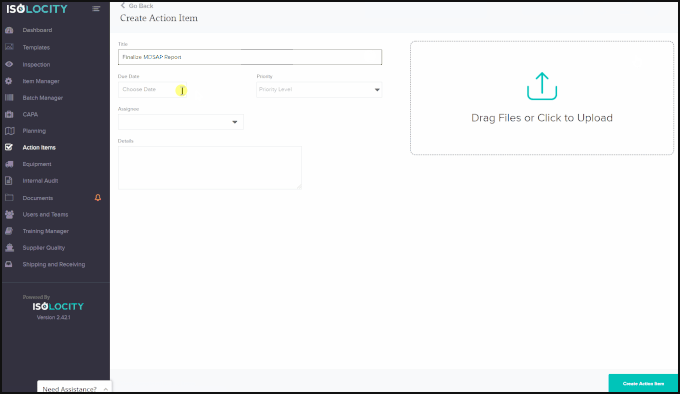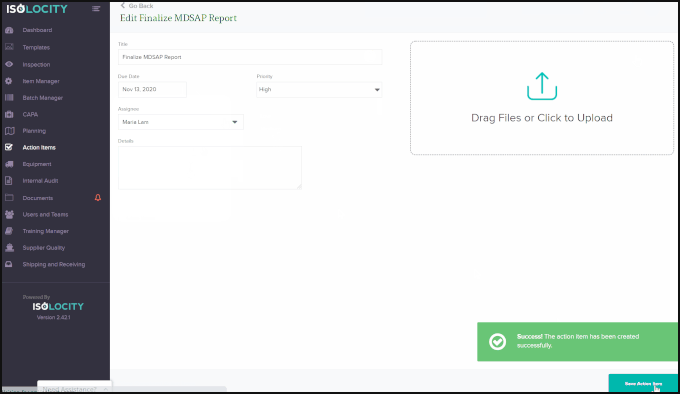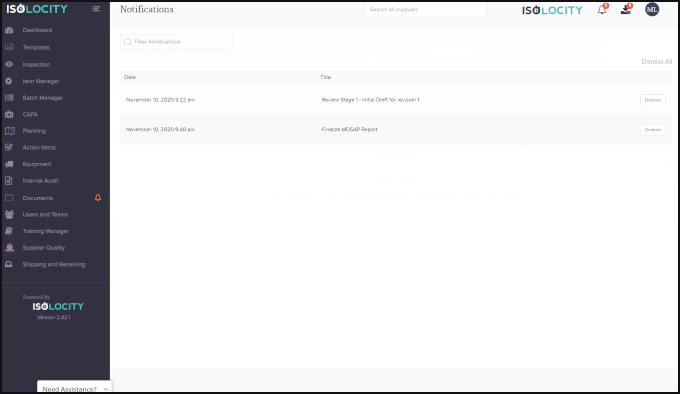
Isolocity’s automation software allows you to devise continuous protocol that maintains quality for you. You can create inspection criteria for ongoing product inspections, distribute employee training, and schedule internal audits as well as regular equipment maintenance in advance. Action items can also be used to assign specific tasks related to MDSAP maintenance as well.
Optimization
Optimization is an essential part of MDSA certification. During the optimization process, you need to market your brand and promotional benefits, as well as optimize your commercial teams. You need to make sure all shareholders and stakeholders are aware of the different processes of the company as they occur.
The Readiness Checklist
The MDSAP readiness checklist is essential because it helps you determine if your company is on track to being successful with the current transition. However, with a QMS like Isolocity some steps can be simplified and automated for continuous improvement.
Getting Your Company Ready
By using GAP analysis, your company can better prepare for new and upcoming organization standards. The MDSAP audit model uses an audit sequence of four primary processes:
- Management
- Measurement, analysis, and improvements
- Design and development
- Production and service controls
The MDSAP audit process provides two different supporting processes, which are device marketing authorization and facility, and medical device adverse events and advisory notices reporting.
Certification Document Specifics
The MDSAP certification document has many different specifics and guidelines.
Format
The format of an MDSAP certification document fits on U.S. letter paper (8.5 x 11 in.). ISO A4 paper can also be used for this certification document. Portrait or landscape printing orientation is permitted unless a participating regulatory authority states otherwise.
Language
MDSAP certification documents are issued in English. Copies of this certification document in different languages is permitted but the officially certified document must be in English.
Identification Code
There is a unique identification code provided for and printed on each certified MDSAP document. This unique identification code’s sole purpose is to identify the certification document. The certification document does not represent a facility, client, or registration.
Document Dates and Validity
Each certification document for MDSAP provides an international year-month-day format. This certification document contains an effective date and an expiry date. The maximum validity period for MDSAP certification documents is three years.
Manufacturer’s Name
All MDSAP certification documents must use the legal name of the manufacturer the certification is issued. Manufacturers name on device labels must match the name on the document.
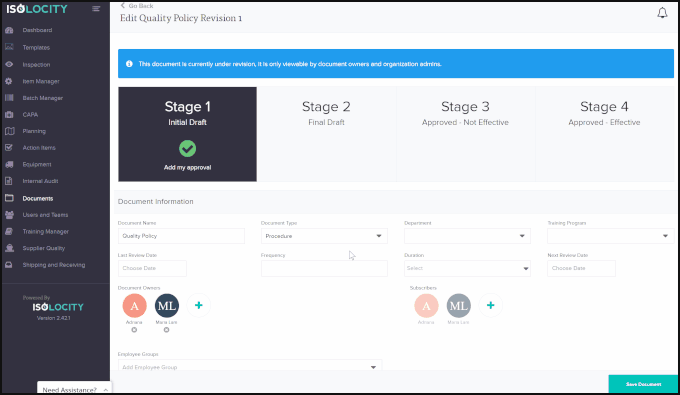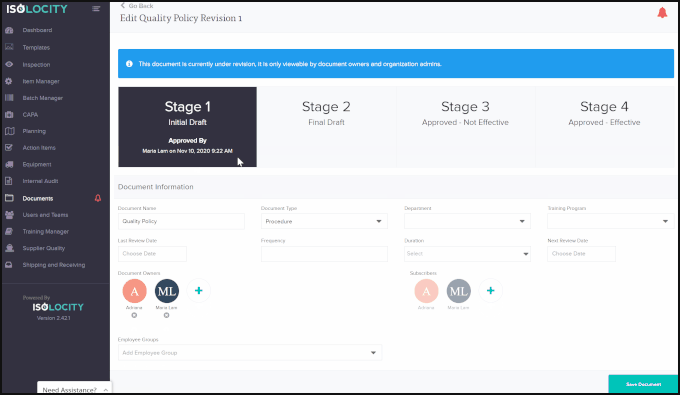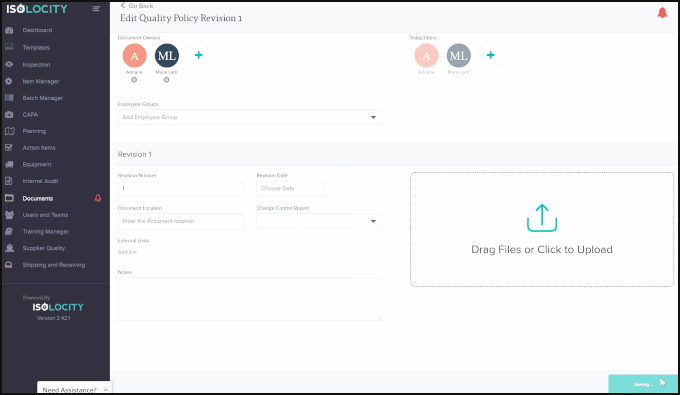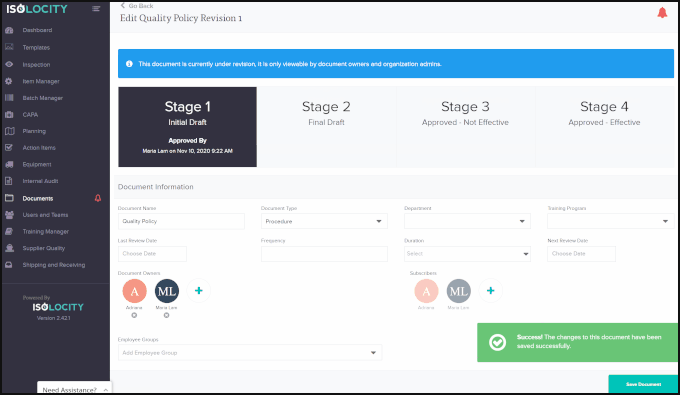
Using Isolocity’s Document Control module your organization can ensure all requirements are followed by having team members make changes and approve the file step-by-step before it is ready to go live. Our approval system uses FDA 21 CFR Part 11 compliant e-signatures for this process.
MDSAP and MDSAP Audit Effectiveness
Patient Safety and Product Quality
Before MDSAP, the accounting for each sector was an overwhelming and nearly an impossible task. Due to these circumstances, businesses performed their audits on a sample basis. Now with the MDSAP, there are more resources available and they can audit areas of their business they were not able to prior to MDSAP.
Enhancing Standard Audits
The MDSAP audit model guarantees consistent processes through evaluation, processing, and assessments. The MDSAP’s audit model and supporting resources such as checklists, help save ample time during the preparation, planning, and execution processes.
Improvements with Resource Management
The MDSAP audit model enables medical device manufacturers to endure only one audit for regulatory purposes instead of three or more separate audits, which saves a lot of time and effort.
The MDSAP has members who network and come together to help their business operations and similar instances associated with their business flow as smoothly as possible. This audit model improves business functionality for many industries.
Here at Isolocity, we proudly provide quality management software that helps your business with ISO and GMP compliance. We are here to help you make all the effort you put into your business work for you. We are not a one-size-fits-all company. All businesses are unique, and we look forward to helping your company with their unique needs. Sign up today for your free trial!