The Hazard Analysis and Critical Control Points (HACCP) system is a leading example of systematic risk management in the field of food safety and quality assurance. Although it originated from NASA in the late 1950s, HACCP has become a structured framework adopted worldwide by companies for recognizing, assessing, and managing risks in food manufacturing processes. Transitioning to discussing the 7 steps of HACCP, it’s one of the most fundamental aspects of this system. Today, we will delve into it in detail.
Prefer video over text? Check this out!
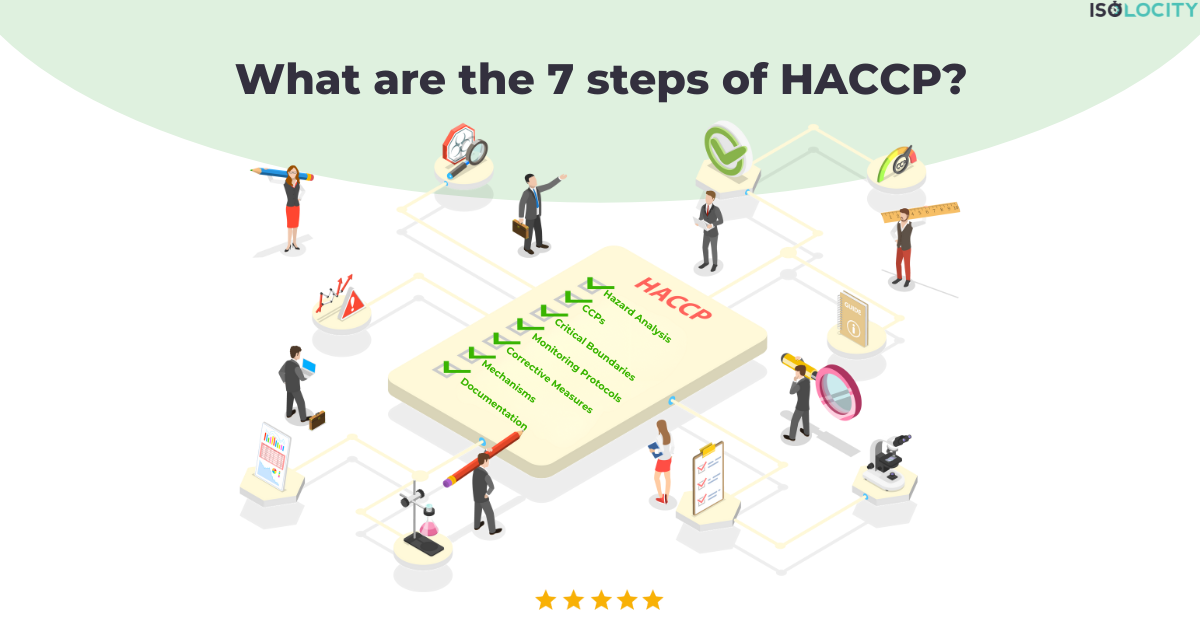
In this blog, we will discuss all the 7 steps of HACCP in detail. But we will also look at all the sub-steps, go through some examples, and ultimately reveal how quality management software like Isolocity can be of great help for HACCP. The 7 steps of HACCP are: 1) Carry out a Hazard Analysis; 2) Identify the Critical Control Points; 3) Define Critical Boundaries; 4) Put Monitoring Protocols into Practice; 5) Decide on Corrective Measures; 6) Check the Mechanisms; and 7) Start Record-Keeping and Documentation.
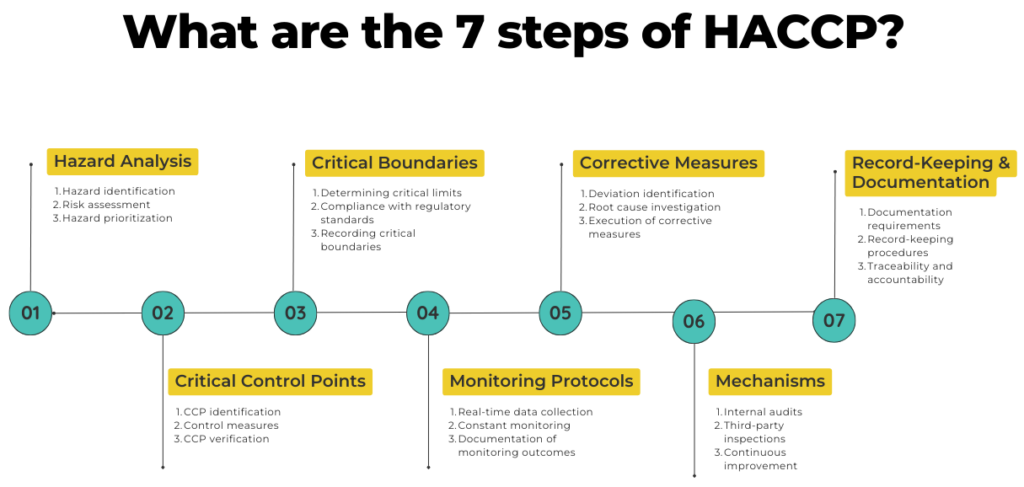
Let’s discuss them in detail.
7 steps of HACCP
1. Carry out a Hazard Analysis
Firstly, performing a hazard analysis is the main component of the HACCP system. Generally, this stage involves the following sub steps:
Sub Steps:
- Hazard identification: Systematically analyzing the production process to identify potential physical, chemical, or biological risks.
- Risk assessment: Determining the likelihood and severity of identified hazards.
- Hazard prioritization: Allocating resources to address the most significant concerns.
E.g.
In a meat processing facility, microbiological contamination from slaughter, chemical contamination from cleaning supplies, and physical hazards from moving parts are all potential risks.
2. Identify the Critical Control Points (CCPs)
Secondly, determining important control points in the production process where control measures can prevent, eliminate, or reduce hazards to acceptable levels involves:
Sub Steps:
- CCP identification: Identifying stages in the production process where control measures are critical.
- Control measures: Establishing processes at CCPs to manage hazards effectively.
- CCP verification: Ensuring the effectiveness of control methods in reducing hazards.
E.g.
In a seafood processing facility, cooking temperatures can be a critical control point where temperature monitoring and time-temperature records help prevent bacterial contamination.
3. Define Critical Boundaries
Determine the metrics and the appropriate instruments for measurement. Monitoring the critical control points ensures that the process remains within the specified critical limits at every juncture.
Sub Steps:
- Determining critical limits: Setting parameters such as temperature, pH, or time within which control measures must operate.
- Compliance with regulatory standards: Ensuring adherence to industry best practices and regulatory requirements.
- Recording critical boundaries: Documenting critical limits for reference and compliance verification.
E.g.
Critical limits for pasteurization in a dairy processing facility might include maintaining a minimum temperature of 72°C for at least 15 seconds to eliminate harmful bacteria.
4. Put Monitoring Protocols Into Practice
Implementing monitoring procedures to verify compliance with critical limits involves:
Sub Steps:
- Real-time data collection: Gathering information on process variables such as pressure, pH, and temperature.
- Constant monitoring: Regularly monitoring CCPs for deviations from critical thresholds.
- Documentation of monitoring outcomes: Recording monitoring data for analysis and confirmation.
E.g.
In beverage bottling plants, filling line temperatures may be continuously monitored to ensure adherence to pasteurization regulations.
5. Decide on Corrective Measures
Developing processes to address departures from critical limits and prevent the creation of unsafe products includes:
Sub Steps:
- Deviation identification: Quickly identifying departures from critical limits using monitoring systems.
- Root cause investigation: Examining underlying causes of deviations.
- Execution of corrective measures: Implementing actions to address root causes and prevent recurrence.
E.g.
A bakery might recalibrate temperature controls or adjust baking durations in response to oven temperature discrepancies.
6. Check the Mechanisms
Validating the efficacy of the HACCP plan through continuous verification processes and performance tracking involves:
Sub Steps:
- Internal audits: Regularly reviewing HACCP records, practices, and controls.
- Third-party inspections: Engaging external auditors or government organizations to assess HACCP compliance.
- Continuous improvement: Identifying opportunities to enhance the HACCP system based on feedback and verification results.
E.g.
Internal audits and third-party inspections in a food packaging facility help evaluate compliance with regulatory standards.
7. Start Record-Keeping and Documentation
Lastly, maintaining thorough records of all HACCP processes is crucial and involves:
Sub Steps:
- Documentation requirements: Identifying industry standards and legal obligations for HACCP documentation.
- Record-keeping procedures: Maintaining accurate and current records of all HACCP activities.
- Traceability and accountability: Using documentation techniques to ensure product traceability and accountability for HACCP implementation.
E.g.
A food distribution center may keep records of shipping, temperature monitoring logs, and ingredient inspections to ensure compliance with HACCP protocols.
How can Isolocity help with HACCP?
Isolocity’s software is tailored to meet the requirements of the food and beverage industry, offering features designed to simplify the implementation of HACCP. With the enhanced planning module, users now have access to a HACCP Risk Report feature, facilitating the creation of HACCP plans. Follow the steps outlined below to utilize this functionality effectively.
-
From the top menu, click on “Create” and then click on “HACCP Risk Management.”
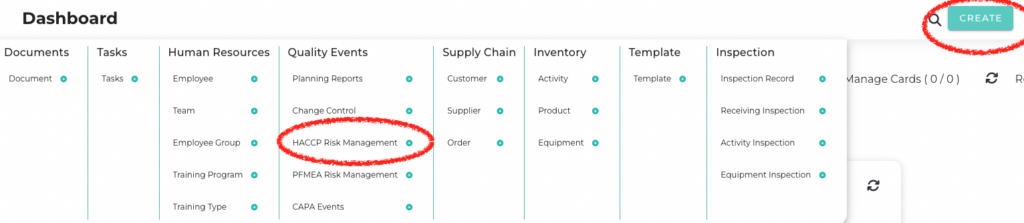
-
Fill out all the necessary fields, which includes
-
Administrative Details
-
Report Number
-
Plan Number
-
Revision Number
-
Form Number
-
Company Name
-
Package Size (whole numbers)
-
Part of Product (should come from part or product, this is a search field)
-
-
HACCP Items
-
Process Steps and Hazard Information
-
Process Steps
-
CCP Hazard Number
-
Hazard Type
-
Chemical
-
Physical
-
Biological
-
Other
-
-
-
Description, Critical Limits and Monitoring Procedures
-
Hazard Description
-
Critical Limits
-
Monitoring Procedures
-
-
Deviation Procedures, Verification Procedure and HACCP Records
-
Deviation Procedures
-
Verification Procedures
-
HACCP Records
-
-
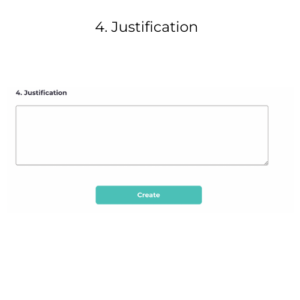
Justification
-
-
-
Click on the “Create” button
-
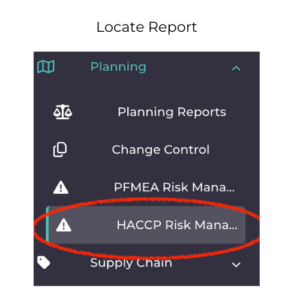
After creation, you will be able to locate it on the left hand menu under the “Planning” module. Click on HACCP Risk Management, and you will come across all HACCP reports. You can either locate it from the list or use the search button or filter option to find your specific report.
- Administrative Details
- 1. Process Steps and Hazard Information
- 2. Description, Critical Limits and Monitoring Procedures
- 3. Deviation Procedures, Verification Procedures and HACCP Records
- 4. Justification
- Locate Report
Conclusion
The 7 steps of HACCP represent a systematic approach to ensuring food safety and quality throughout the production process. By leveraging Isolocity’s quality management software, organizations can streamline each step of the HACCP system, from hazard analysis to documentation and record-keeping, enhancing efficiency, accuracy, and compliance. As the global food industry continues to evolve and face new challenges, Isolocity’s innovative solutions empower organizations to uphold the highest standards of excellence in food safety and quality assurance, safeguarding consumer health and trust in the process.