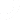Finding ways to optimize quality control is important. In fact, it should be one of the top priorities of all high-performing companies. Even the smallest companies are now competing against big corporations. Therefore, ensuring that one’s products are always of optimal quality is imperative to the overall success of your company.
Consumers have to be more selective in their spending so only quality products will maintain a share of the wallet. This means that companies must make sure they are staying on top of quality control. Orders are high volume and requested from virtually anywhere in the world. This is where the implementation of an EQMS (Electronic Quality Management System) can make a difference.
What is PFMEA?
Simply put, PFMEA or process failure mode effects analysis, refers to a process used to identify and rate the critical failure modes within the manufacturing process. PFMEA can uncover potential issues and address them before they are major problems in the manufacturing process. Therefore, it is most useful during the design phase. The process uses data collected as well as charts and diagrams. This allows for the visual representation of the weaknesses and assesses how the issues can be addressed.
What is the Purpose of PFMEA?
As mentioned, PFMEA is used to uncover weaknesses throughout the organization’s process. This helps to ensure both optimal efficiencies as well as the overall safety of the products being created. The vaping industry deals with tobacco products and can be dangerous if not properly constructed. This makes it especially important for manufacturers to stay on top of quality control at all times. PFMEA allows companies to keep a proverbial microscope on the ins and outs of the process. Additionally, it makes this complex process simpler and more accurate.
Economies have been shifting toward remote work for almost a decade but the emergence of new health risks has forced organizations to quickly adapt touchless production procedures to fulfil their compliance obligations and keep their doors open. This can be avoided by implementing an EQMS with a PFMEA module. The PFMEA should consider an organization’s ability to reduce contamination and work remotely while keeping the workforce healthy. An EQMS reduces human contact using digital forms and other circulated documents which include e-signature change control procedures, which mitigates these risks.
What do You need to Meet its Requirements?
To operate PFMEA effectively, here are the requirements:
- A Team of Professionals- A company concerned with quality control must employ those who are familiar with the ins and outs of the process.
- A Cross-Functional Team- A company must have a team that includes a combination of both those new to the field and seasoned veterans. This is necessary in order to be able to truly analyze all elements of the process from varying perspectives. Therefore, companies lacking either young, inexperienced employees or older experts are at a disadvantage with regards to PFMEA. As far as the vaping industry is concerned, this makes PFMEA a perfect fit.
- A Thorough Analysis Process- Many industries tend to create and produce products that include intricate parts and technology. Therefore, companies must make sure that each part of the process is functioning properly. This ensures a safe product is sold to the masses.
Using PFMEA For Risk Management In Isolocity
Automation is the future of quality control. It was a given that companies would spend thousands of dollars annually, to get a handle on quality control. Fortunately, this is no longer necessary for success. Using quality management software, companies can now limit the amount of time and resources they are putting into managing quality control. Moreover, there are ways to make the process quicker and more accurate. For instance, using Isolocity’s PFMEA quality management software is one of the best options for companies who are seeking the best methods of handling quality control.
You can add core team members as owners or subscribers to any PFMEA report and have them follow through with analysis or actions from directly within the risk planning module:
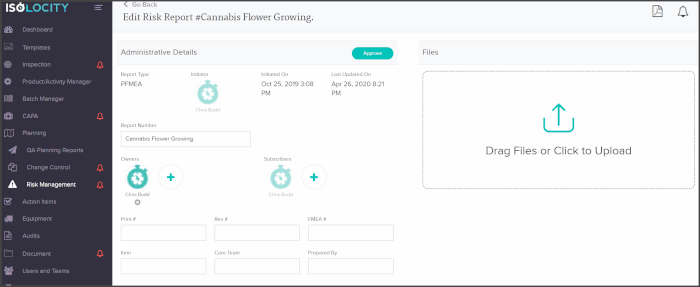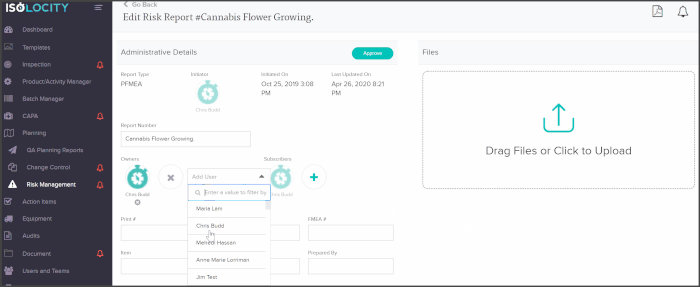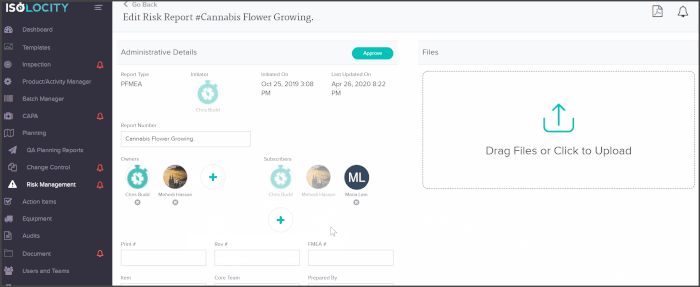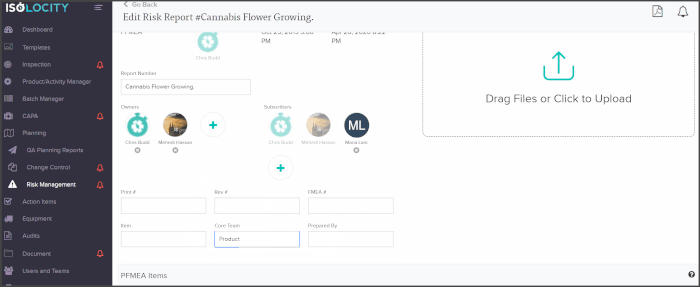
The PFMEA template is ready for users to just fill out. The system will even calculate the severity, detection and RPN levels based on these inputs:
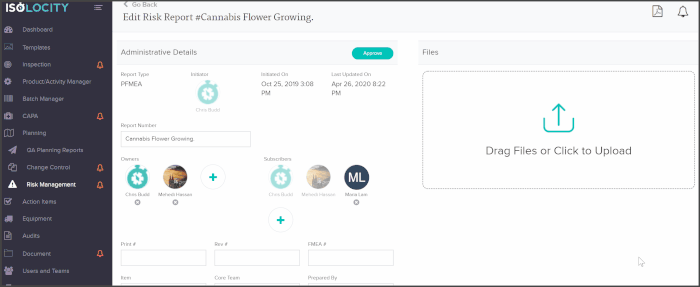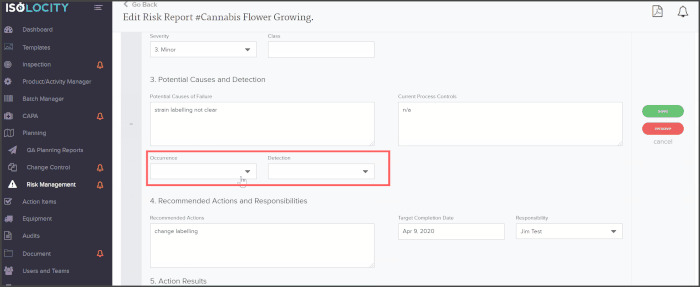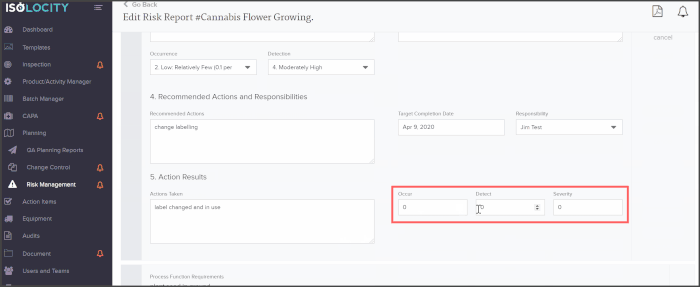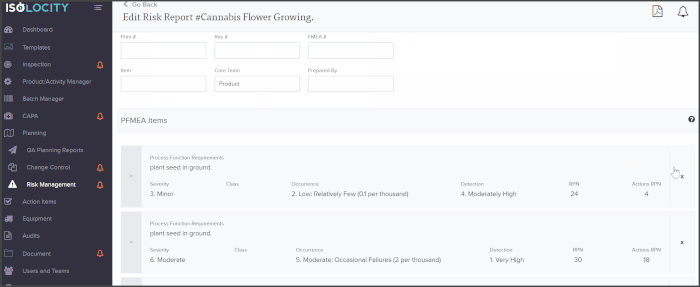
Ask us about how you can implement PFMEA with Isolocity’s EQMS today. Our cloud-based quality management system is ready to help you manage risks, CAPA, inspections, and more whether you’re in the office or working remotely.