The scope of a quality management system is one of the most important aspects to consider while implementing it. It helps in deciding what needs to be included and excluded from the quality management system. The scope is defined by identifying the products, services, and processes that are covered by the QMS for achieving the desired results. This article, along with the process of deriving the scope of quality management system also provides a scope of quality management system PDF template (and word doc too). Download the same from here.
Looking for a cloud based quality management system? Talk to our product specialist.
Now you can watch this blog too!
scope-of-quality-management-system (Scope of quality management system Word template)
scope-of-quality-management-system (Scope of quality management system PDF template)
How to derive the scope of Quality Management System
What is the scope?
The scope of the quality management system is the boundaries of your business. It is a set of activities, products and services that make up your organization.
You need to know what the scope is before you start designing a quality management system for your business because you will use this knowledge when creating policies and procedures for your system.
It’s important that everyone in the company understands their role in maintaining their work area so that nothing gets missed or overlooked during regular inspections, which ultimately results in better customer satisfaction rates due to fewer errors being made during production cycles with costly consequences if they aren’t caught early enough!
How can we define the scope?
Once you’ve defined your quality management system, it’s time to define your scope. The scope is the boundary for what is considered part of the quality management system and what isn’t. To define it, you need to look at three things:
- What are our organizational boundaries? For example, does the organization operate in more than one country or state? Are there multiple facilities that make up the company?
- What do we produce or deliver as a product or service? Do we offer multiple products or services with different characteristics from each other (like a restaurant with different types of menu items)?
- How much variation can be expected in terms of products/services produced within a reasonable timeframe or period of time (for example, manufacturing automobiles)?

Main factors of the scope of quality management system
The scope of quality management system is a document that defines the boundaries and limitations of your quality management system. It helps to ensure that you are following best practices and prevents you from wasting time, money and resources trying to meet requirements that have not been identified yet. A good scope will also tell you what is not included in your quality management system so that everyone knows what they can expect from it.
A well-defined scope should provide answers to all these questions:
- What does your company do? Who are its main customers? What are their needs? What kind of operations does it perform? How does this relate back to the overall purpose of the company (e.g., providing excellent customer service)?
- Why does your organization need a QMS? Are there any regulatory issues that require compliance with specific standards/guidelines for example ISO 9000 series). Do any customers require such control systems as a condition for doing business with them (e.g., some government agencies may require contractors working on projects funded by them).

Are all requirements applicable to our organization as per the ISO standard, identifying what is not applicable?
As you begin to gather information about the scope of your quality management system, it’s important to remember that ISO has a lot of requirements. You’ll need to evaluate each requirement carefully and determine its applicability to your organization. It’s also important to identify what is not applicable at this time. For example, if there are specific standards that apply only under certain conditions or in relation to particular types of products or services, those will have no relevance for your organization right now. What’s more is that there may be other requirements that are not relevant because they are not practical or achievable with the resources available within your company (e.g., conducting audits).
Once you’ve identified which requirements are relevant and which ones aren’t, you’ll then want to prioritize them based on their importance for operationally effective quality management systems (i.e., QMSs).
Should the internal audit be conducted to ensure that all aspects have been considered and covered or not?
The answer is no. A detailed internal audit is not mandatory, but it is a good practice to perform one.
The purpose of conducting an internal audit is to ensure that all aspects have been considered and covered in your quality management system (QMS), which will help you maintain compliance with your own QMS regulations and standards such as ISO 9001:2015, ISO 13485:2016 etc.
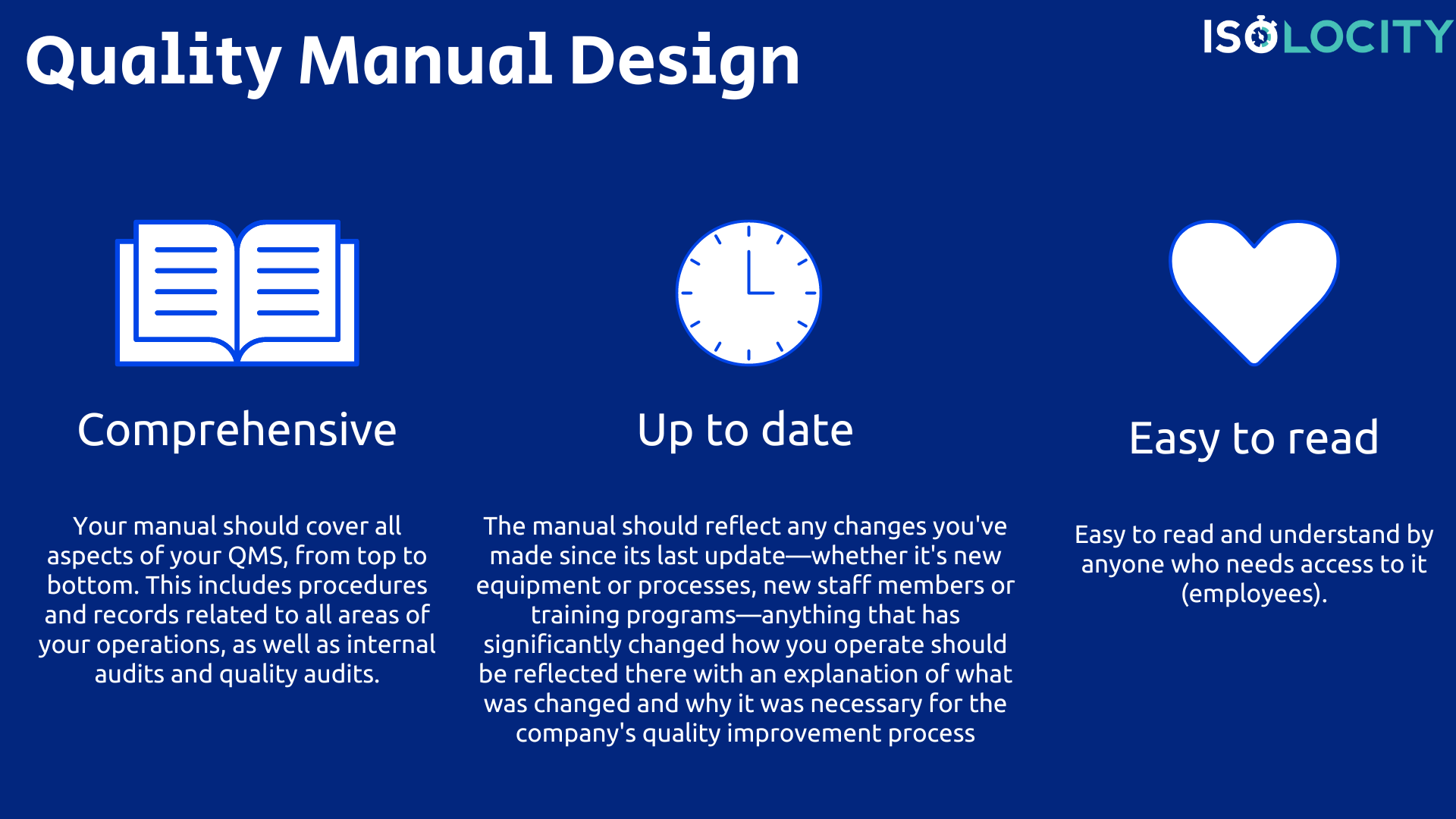
How should you design your Quality Manual?
The Quality Manual is a document that provides in-depth information on how the QMS functions. It should be:
- Your manual should cover all aspects of your QMS, from top to bottom. This includes procedures and records related to all areas of your operations, as well as internal audits and quality audits.
- Completely up to date. The manual should reflect any changes you’ve made since its last update—whether it’s new equipment or processes, new staff members or training programs—anything that has significantly changed how you operate should be reflected there with an explanation of what was changed and why it was necessary for the company’s quality improvement process.
- Easy to read and understand by anyone who needs access to it (employees).
What should be included in the Procedure Manual?
The Procedure Manual should be used by employees to help them do their job. It should include the following:
- An explanation of the Quality Management System (QMS) and how it works, including any relevant standards and regulations that apply
- A list of quality management documents, including QMS policies, procedures and work instructions (WIs)
- A list of any quality-related documents that are referenced in your SOPs for easy access
Conclusion
The scope of quality management system is a very important part of the entire process. It helps in identifying and understanding what is required to be included in the Quality Management System. We hope the scope of quality management system PDF and word template will help you design the document. For any doubt, feel free to reach out to us.
Template original source credit: stakeholdermap.com